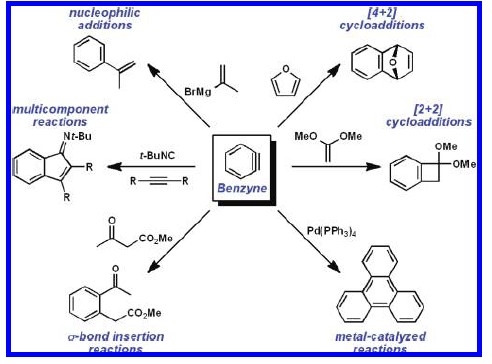
Within 14 years of the seminal experiments of J. D. Roberts leading to the first proposal of the structure of benzyne (1),synthetic organic chemists recognized the potential to exploit this highly reactive intermediate (and its substituted variants) in the total synthesis of natural products. More specifically, it was recognized that arynes offered the strategic advantage of rapidly functionalizing an aromatic ring by forming multiple carbon−carbon or carbon−heteroatom bonds in a single operation, of ten in a regioselective manner. Initially, the scope of synthetic applications was somewhat limited by the harsh conditions required to produce the aryne species.2 Many of these methods required strong bases, such as n-BuLi, or high temperatures (Scheme 1). However, with the development of milder methods for the generation of arynes came increased interest in employing them in the synthesis of more complex polycyclic systems. Most recently, the use of o-silyl aryl triflates as aryne precursors has allowed generation of the reactive intermediate under almost neutral conditions.
 AComprehensiveHistoryofArynesinNaturalProductTotal.pdf
AComprehensiveHistoryofArynesinNaturalProductTotal.pdf

